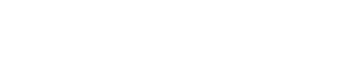The FORTIFI-HN01 clinical trial is for people with Head and Neck Squamous Cell Carcinoma (HNSCC) that has either returned (recurrent) or spread to other parts of the body (metastatic).
What you need to know about the FORTIFI HNSCC Clinical Trial
The FORTIFI clinical trial is evaluating an investigational medication called ficerafusp alfa in patients with
Head and Neck Squamous Cell Carcinoma
(HNSCC) that has either returned or spread to other parts of the body (recurrent or metastatic HNSCC). This type of cancer arises from the squamous cells lining the moist mucosal surfaces in the head and neck region, including the oral cavity (mouth), pharynx (throat), and larynx (voice box).
The study drug, ficerafusp alfa, combines 2 different proteins that may help stop messages that contribute to tumor growth from being sent.
You can view this site on clinicaltrials.gov, using the study identifier FORTIFI
Why should I participate in the FORTIFI
Clinical Trial
- About 650 people with recurrent or metastatic head and neck squamous cell cancer are expected to take part in this study. The hope is that the study drug in combination with pembrolizumab will help the body’s immune system to better fight and treat cancer cells than pembrolizumab alone.
- Everyone participating in the clinical trial will receive pembrolizumab (a type of immunotherapy approved by the FDA for treating head and neck cancer). Most patients will also receive the study drug in addition to pembrolizumab.
- A team of doctors and nurses will monitor your health carefully during the study. The study treatment (study drug or placebo) and all study-related tests will be provided at no cost to you. You can choose to stop your participation in the study at any time.
Am I eligible for the HNSCC trial?
Men or women 18+ years of age or older
Have been diagnosed with recurrent or metastatic head & neck cancer HNSCC
Completed prior therapy more than 6 months ago before cancer was recurrent or metastatic
If you answered YES to these questions, you may be a good candidate for this trial and should talk to your doctor or contact a trial location listed below
Where is the FORTIFI Clinical Trial taking place?
The trial will take place at several locations across the United States.
Travel expenses, including hotel reimbursement, may be provided for you + a caregiver
Please contact the nearest study center below for additional information.
Alabama
AUB O'Neal CCC
Birmingham, AL 35233
Arizona
Mayo Clinic Arizona
Phoenix, AZ 85054
California
UCLA Westwood
Los Angeles, CA 90095
UCSD Moores Cancer Center
La Jolla, CA 92093
University of California San Francisco
San Francisco, CA 94158
UC Davis Comprehensive Cancer Center
Sacramento, CA 95816
Stanford University
Stanford, CA 94305
Colorado
Rocky Mountain Regional VA Medical Center
Aurora, CO 80045
University of Colorado Anschutz Medical Campus
Aurora, CO 80045
Rocky Mountain Cancer Centers
Aurora, CO 80012
Delaware
Christiana Care
Newark, DE 19713
Florida
Moffitt Cancer Center
Tampa, FL 33612
Cancer Care Centers of Brevard
Palm Baya, FL 32909
Mayo Clinic
Jacksonville, FL 32224
Iowa
University of Iowa/Holden Comprehensive Cancer Center
Iowa City, IA 52242
Kansas
The University of Kansas Cancer Center
Westwood, KS 66205
Illinois
Captain James A. Lovell Federal Health Care Center
North Chicago, IL 60064
Kentucky
Norton Cancer Institute
Louisville, KY 40202
University of Kentucky Markey Cancer Center
Lexington, KY 40536
UofL Health - Brown Cancer Center
Louisville, KY 40202
Massachusetts
Dana-Farber Cancer Institute
Boston, MA 02135
Massachusetts General Hospital, Mass General Brigham Cancer Institute
Boston, MA 02114
Maryland
University of Maryland
Baltimore, MD 21201
Minneosotta
Mayo Clinic Rochester
Rochester, MN 55905
Missouri
Washington University School of Medicine
St. Louis, MO 63110
North Carolina
Duke University Health System
Durham, NC 27710
New Jersey
John Theurer Cancer Center at Hackensack University Medical Center
Hackensack, NJ 07601
New York
Memorial Sloan Kettering Cancer Center
New York, NY 10065
Ohio
Cleveland Clinic
Cleveland, OH 44195
University of Cincinnati Medical Center
Cincinnati, OH 45267
University Hospitals Cleveland Medical Center
Cleveland, OH 44106
Cleveland Clinic
Canton, OH 44708
Oregon
Providence Cancer Institute - Franz Clinic and Portland
Portland, OR 97213
Philadelphia
UPMC Hillman Cancer Center
Pittsburgh, PA 15232
VA Pittsburgh Health System
Pittsburgh, PA 15240
Rhode Island
Brown University Health
Providence, RI 02903
VA Pittsburgh Health System
Pittsburgh, PA 15240
South Carolina
Medical University of South Carolina
Charleston, SC 29425
Tennessee
Vanderbilt-Ingram Cancer Center Clinical Trials Office (CTO)
Nashville, TN 37232
SCRI-Oncology Partners
Nashville, TN 37203
Texas
UT MD Anderson Cancer Center
Houston, TX 77030
Texas Oncology Central-South
Waco, TX 76712
Virginia
University of Virginia
Charottesville, VA 22908
Virginia Commonwealth University
Richmond, VA 23298
Washington
Fred Hutch Cancer Center
Seattle, WA 98109
Northwest Cancer Specialists, PC
Vancouver, WA 98694
Wisconsin
William S. Middleton Memorial Veterans' Hospital
Madison, WI 53705
*Additional locations opening soon
Frequently Asked Questions
What is a clinical trial?
A clinical research study is a medical study that helps to answer important questions about an investigational medication, such as:
• Does it work?
• How safe is it?
• What are the side effects?
Clinical studies play a key role in the fight against cancer. Through these studies, we can explore new and potentially better ways of identifying, diagnosing, and treating cancer, with the goal of improving outcomes for people affected, now and in the future.
How many patients will be enrolled?
Approx 650 patients.
Will I be paid to participate?
The study treatments and all study-related tests will be provided at no cost to you. While there is no payment to take part in the study, support for reasonable travel costs will also be available. Participation in this trial is voluntary.
How is this study designed?
The study is designed to help researchers better understand how safe the study drug is and whether it can stop or slow tumor growth in people with recurrent or metastatic HNSCC.
The study has 2 parts:
Part 1 aims to identify the optimal dose of the study drug when given in combination with an approved medication for recurrent or metastatic HNSCC called pembrolizumab. The optimal dose is the dose at which the study drug works best.
Part 2 aims to find out if the combination of the study drug with pembrolizumab works to stop or slow cancer growth better than pembrolizumab on its own. This part will also find out more about the safety of the optimal dose of the study drug when given in combination with pembrolizumab.
The part you are in will depend on when you join the study. In both parts of the study, you will have a higher chance (2-in-3) of receiving the study drug with pembrolizumab than the placebo with pembrolizumab (1-in-3).
Does ‘placebo’ mean I may not be treated?
All participants will receive pembrolizumab in this study, an immunotherapy approved by the FDA to treat head and neck cancer.
If you participate, you will be randomly assigned to receive the study drug and pembrolizumab or pembrolizumab plus a placebo, a solution that does not contain the study drug. You will have a higher chance (2-in-3) of receiving the study drug with pembrolizumab than the placebo with pembrolizumab (1-in-3).
How long will the study last?
You may receive treatment on the study as long as you are benefiting from treatment.
There is a safety follow-up period for approximately 3 months after your last dose of the study treatment.
There is a long-term follow-up period where the study team will contact you by phone every 3 months and ask you about your health and whether you are receiving any new cancer treatments.
Where does the study take place?
The FORTIFI-HN01 study is global, taking place in numerous countries around the world. The United States has multiple cancer centers currently enrolling participants in this trial.
How are the medications given in the study?
The study drug, the placebo, and pembrolizumab will be given as intravenous infusions, through a small tube inserted into a vein in the arm.
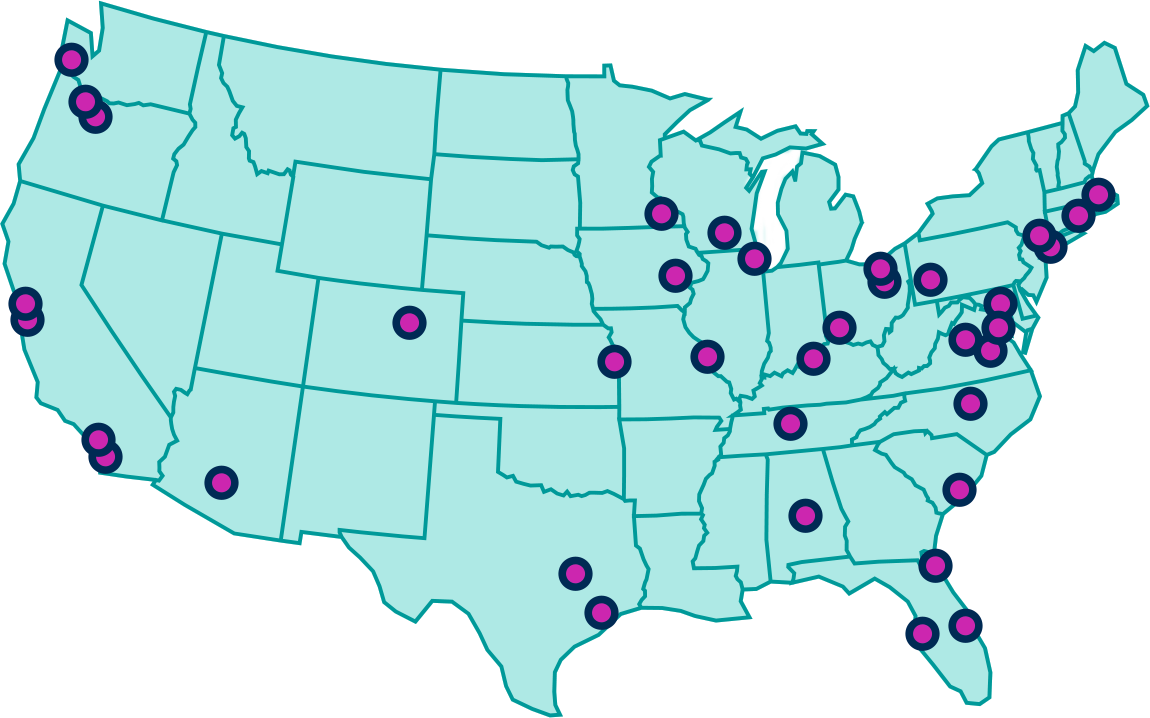
What is ficerafusp alfa and how does it work?
Ficerafusp alfa is an investigational drug designed to help the immune system more effectively reach and attack certain tumors, including head and neck cancers. Many solid tumors build “barriers” around themselves that make it hard for the body’s immune system and current treatments to get inside and do their job.
Ficerafusp alfa is designed to help with two important things:
1. Finding the cancer cells
It attaches to a protein called EGFR, which is often present on the surface of certain cancer cells. Think of EGFR as a “flag” on the cancer cell that helps the medicine locate the tumor.
2. Breaking down the tumor’s protective shield
Many tumors create a thick, tough environment using a substance called TGF- β, which makes it harder for immune cells to enter. Ficerafusp alfa has a second built in function that blocks TGF- β, helping “open up” this barrier so treatments and immune cells can get in.
Has ficerafusp alfa been studied in head and neck cancer before?
Yes. Ficerafusp alfa has already been tested in Phase 1 clinical studies with participants who have HNSCC. Early trials have shown encouraging results, including deep and durable (long lasting) responses when combined with pembrolizumab, a drug already approved by the FDA and other health authorities for use in patients with recurrent or metastatic HNSCC. These early trials also evaluated the safety of the study drug when combined with pembrolizumab. The adverse events (side effects) were generally well-tolerated with no treatment-related deaths. Because these early trials were small, the larger Phase 2/3 FORTIFI-HN01 study is now underway to confirm these results in a broader group of patients.
The early findings also led the FDA to grant ficerafusp alfa ‘Breakthrough Therapy Designation’ for first line treatment of HPV- negative recurrent or metastatic HNSCC. Breakthrough Therapy Designation is a special status that the FDA gives to experimental medicines that show very promising early results for serious or life-threatening conditions. It helps patients get access to potentially important new treatments as quickly and safely as possible.
Can I choose which treatment I receive?
Participants will be randomly assigned (by chance) to receive one of the combinations. You will have a higher chance (2-in-3) of receiving the study drug with pembrolizumab than the placebo with pembrolizumab (1-in-3). This study is blinded, which means that the patient and the doctor do not know which combination is being administered. Blinding helps make sure the results show what the treatment really does, without opinions or guesses getting in the way.
I’m interested. How do I take the next step?
Talk to your doctor to find out if you are a good candidate for this study. You can also contact one of the study locations listed. Additional details about this study are available on clinicaltrials.gov
Phase 1 Data in HPV-neg 1L R/M HNSCC, PD-L1 CPS ≥1 (NCT06788990)
The single-arm cohort of ficerafusp alfa 1500 mg IV QW with pembrolizumab 200 mg IV Q3W was presented at ASCO 2025 4
54% confirmed overall response rate, 21% complete response
21.7 months median duration of response
9.9 months progression-free survival
89% disease control rate
21.3 months median overall survival
Complete and prolonged neutralization of TGF-β1 was observed
The single-arm cohort of ficerafusp alfa 750 mg IV QW with pembrolizumab 200 mg IV Q3W was reported at ESMO Asia 2025
5
Link to Slide >>
57% confirmed overall response, 7% complete response
83% disease control rate
Complete and prolonged neutralization of TGF-β1 was observed
Safety
The combination was tolerable with a consistent and manageable safety profile. No treatment-related deaths were reported in either cohort. The most frequent treatment-related AEs (any grade) in both cohorts were acneiform dermatitis and pruritus. Additional safety tables available in ASCO 2025 and ESMO Asia 2025 presentations on
Bicara.com/science
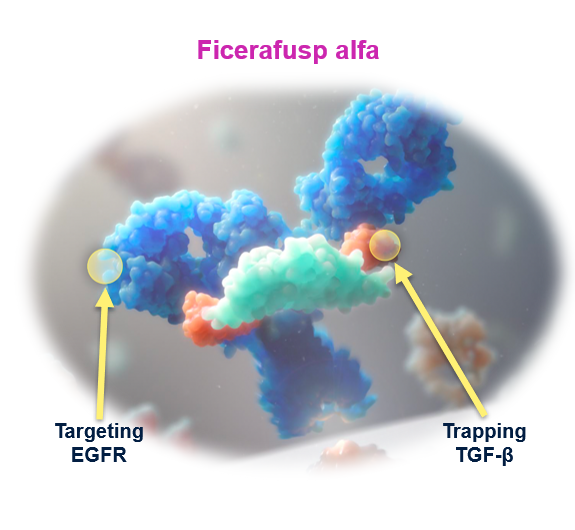
FORTIFI HN-01 Study Overview
Brief Summary
Ficerafusp alfa is directed against two targets, Epidermal Growth Factor Receptor (EGFR) and Transforming Growth Factor beta (TGF-β).
This study is designed to evaluate the safety and efficacy of ficerafusp alfa in combination with pembrolizumab versus placebo with pembrolizumab in 1L HPV-negative, PD-L1-positive, recurrent or metastatic Head and Neck Squamous Cell Carcinoma (HNSCC).
FORTIFI-HN01 Detailed Description
Design and Objectives Phase 2/3, seamless, global, multicenter, randomized, placebo-controlled, double-blind study
Phase 2 of the study will identify an optimal biologic dose (OBD) supported by safety, tolerability, PK, PD, and efficacy data of ficerafusp alfa. In this part, eligible subjects will be randomized to one of three treatment arms at a 1:1:1 ratio:
- Arm A: ficerafusp alfa 1500 mg once weekly (QW) + pembrolizumab 200 mg every three weeks (Q3W).
- Arm B: ficerafusp alfa 750 mg QW + pembrolizumab 200 mg Q3W.
- Arm C (control): placebo QW + pembrolizumab 200 mg Q3W.
The primary objective for the phase 3 portion is to compare the efficacy in subjects treated with ficerafusp alfa at the selected OBD in combination with pembrolizumab versus placebo with pembrolizumab. Eligible subjects will be randomized 2:1 in the treatment versus control arm during the phase 3 portion.
Phase 2 outcome measures:
- Incidence and severity of TEAEs, treatment-treatment emergent SAEs TEAEs leading to dose interruption, dose reduction, or permanent discontinuation.
- To assess the safety and tolerability of ficerafusp alfa with pembrolizumab.
- Objective Response Rate (ORR) per RECIST 1.1 by blinded independent central review (BICR).
Phase 3 outcome measures:
- Objective Response Rate (ORR) per RECIST 1.1 by BICR.
- There will be an interim analysis of ORR
- Overall Survival (OS)
Enrollment between phase 2 and phase 3 is seamless, with no pause in enrollment. Randomization is 2:1 for ficerafusp plus pembrolizumab vs pembrolizumab plus placebo for Phase 2 AND Phase 3
Key Eligibility Criteria
Additional criteria available on clinicaltrials.gov
Key inclusion criteria
· Histologically or cytologically confirmed R/M HNSCC
· Eligible primary tumor locations: oral cavity, hypopharynx, larynx, or oropharynx (HPV-negative by central PCR if OPSCC)
· PD-L1 CPS ≥1
· No prior systemic therapy in the R/M setting, and completed systemic therapy >6 months prior if given as part
of multimodal treatment for
· Locoregionally advanced disease
· Measurable disease per RECIST 1.1
· Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1
· Age ≥18 years
Key exclusion criteria
· Disease suitable for local therapy administered with curative intent
· Prior treatment with therapy targeted to TGF-β
· Prior therapy with an anti-EGFR antibody (except for radiosensitizing and multimodal treatment for locoregionally advanced disease)
· Prior neoadjuvant and/or adjuvant immune checkpoint inhibitor therapy completed ≤6 months prior to study treatment initiation
· History of grade ≥2 intolerance or hypersensitivity reaction to anti-EGFR therapy or other murine proteins
Estimated enrollment is 650 participants.
Locations
FORTIFI Clinical Site Locations can be found here >>
This study is taking place in multiple locations within the US and in numerous countries around the world.
References
1. Shen X, et al. Bioact Mater. 2023;26;32:445-72.
2. Minchinton A, Tannock I. Nat Rev Cancer. 2006;6(8):583-92.
3. O’Connell BC, et al. Cancer Res. 2025;85(8_Suppl 1):Abstract 3284.
4. Chung CH, et al. J Clin Oncol. 2025;43(16 suppl):6017.
5. Kaczmar JM, et al. Annals of Oncology (2025) 36 (suppl_4): S2001-S2024.